Cybin Inc. continues researching the efficacy of CYB003, a proprietary deuterated psilocybin analog, for treating people with Major Depressive Disorder. The Positive interim data from their Phase 2 trial shows significant promise–with a “20% remission rate (no longer meeting the clinical definition of depression) vs 0% for the placebo,” all with a favourable safety profile.

Major Depressive Disorder (MDD) is a health condition that casts a dark shadow over the lives of millions of people. The symptoms are persistent and include everything from sadness, feelings of hopelessness, and lack of interest in daily activities. Finding suitable treatments for MDD has been challenging, leaving those affected longing for a breakthrough.
There is now a glimmer of hope on the horizon. Cybin Inc., a clinical-stage biopharmaceutical company, has revealed remarkable interim results from Phase 2 trials of their proprietary deuterated psilocybin analog known as CYB003. Three weeks following a single dose, researchers witnessed an exciting 20% remission rate amongst participants. With this, the primary efficacy endpoint has been met, with full topline data on schedule for Q4 2023 release.
These results do not only provide hope to individuals battling MDD, they also represent a significant shift in how this complex condition could be addressed.
MDD: A Deep-Rooted Crisis
Major Depressive Disorder is a crisis that affects individuals regardless of their background or circumstances. It knows no boundaries and impacts people, across ages, races and walks of life. Its grip continues to undermine the essence of living a fulfilling and healthy life.
Traditionally, MDD has been addressed through a combination of antidepressant medications and psychotherapy. But not everyone can benefit from this one-size-fits-all approach. There is a need for new, innovative treatments in the field of mental healthcare.
Psychedelics; Pursuing an Alternative Solution
For years, scientists and researchers have been exploring alternative approaches to treating MDD. Over time, psychedelics have emerged as an option, as compounds like psilocybin and DMT found in psychedelics have long been associated with mental growth. Since the psychedelic renaissance of the 1960s, there has been increasing curiosity about the potential of these treatments, as they can induce states of consciousness allowing for self reflection and emotional healing–but a complex political landscape, the war against drugs, and other hang ups have slowed clinical research.
But with humanity feeling the pain of mental illnesses like MDD, navigating the regulatory landscape and ensuring the safe and controlled research of psychedelics has never been more important. This is where Cybin comes into play.
The Emergence of Cybin and CYB003
Established in 2019, Cybin Inc. aims to revolutionize healthcare by developing psychedelic-based treatment options for individuals suffering from mental health conditions. Their approach explores the potential of psychedelics within a controlled, rigorous research-based environment.
CYB003, Cybin’s proprietary deuterated psilocybin analog, continues to show promise during Phase 2 trials. Interim data, put out in a recent press release, showed “unprecedented” results: “rapid and significant improvements in depression symptoms after single dose.”
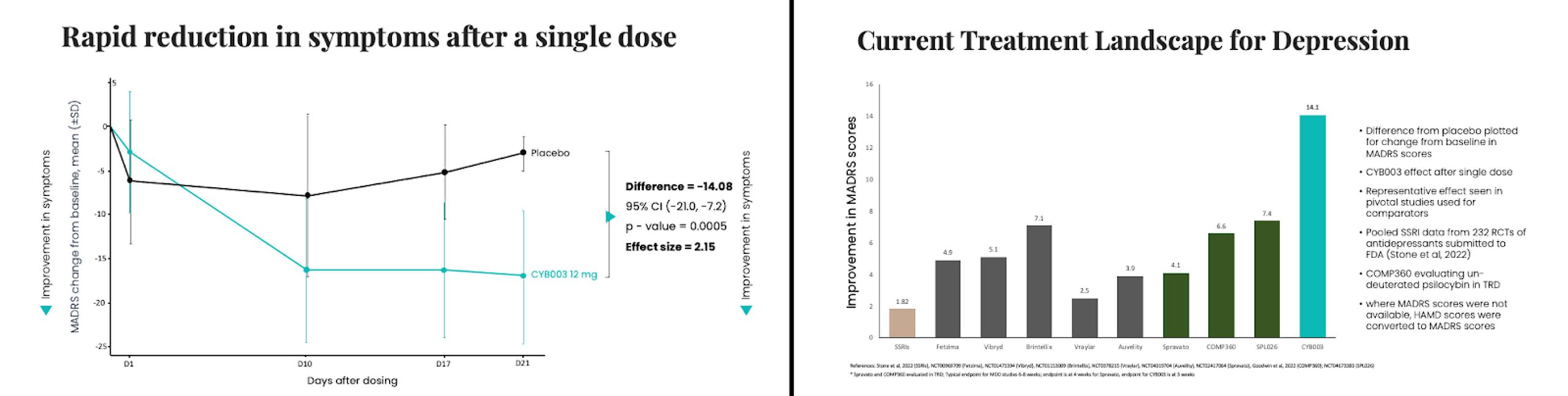
When CYB003 was given as a 12mg dose, it displayed a significant decrease in depressive symptoms compared to a placebo. These findings, supported by a p-value of 0.0005, indicate a difference that marks a step towards improving the treatment of MDD.
Doug Drysdale, the CEO of Cybin, expressed his optimism regarding these results in the press release by stating that the interim findings for the 12mg dosage of CYB003 are incredibly encouraging for patients and healthcare providers. The efficacy demonstrated at this dosage level showcased a reduction in symptoms when compared to currently available treatments.
Although these interim results are promising, they only offer a glimpse into the potential of CYB003. The full set of top line data is expected to be shared this quarter while durability data over a period of 12 weeks is anticipated in Q1 2024. These data points could hold the key to ushering in a new era for MDD treatment. This brings renewed hope to individuals who have struggled with this condition for years.
The Future of Mental Healthcare
The interim efficacy data from CYB003’s Phase 2 trial shed light on the changing landscape of healthcare and the increasing recognition of psychedelic-assisted therapies as a powerful tool for treating various mental health conditions.
This positive progression is not significant for Cybin but for the entire medical sector. As research advances and the potential of CYB003 becomes clearer, it paves the way to explore the properties of psychedelics further.
In addition to CYB003, Cybin is working on CYB004, a DMT molecule for generalized anxiety disorder and has a research pipeline consisting of investigative psychedelic-based compounds. The doors to possibilities in healthcare are gradually opening, with exciting potential for those seeking more effective treatments.
This research marks a new era for both Cybin and the entire field of healthcare. With the potential for a treatment for MDD, these Phase 2 interim results are only the beginning.
Cybin will host a conference call on November 1 2023 to delve deeper into these findings. The company also anticipates releasing Phase 1 data highlights for CYB004 and SPL028 their novel proprietary deuterated DMT compounds before the end of 2023.
For information about Cybin and their groundbreaking research please visit www.cybin.com.
